Virus associated protein
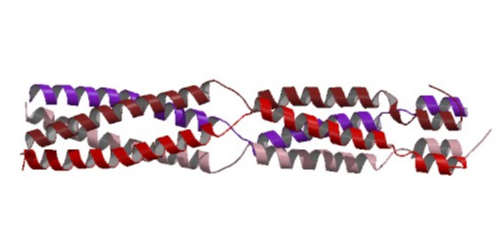
The "virion-associated protein" (VAP) encoded by many but not all caulimoviruses is essential for plant infection (Stavolone et al. 2001), and can participate in the virus movement (Stavolone et al. 2005). VAP contains coiled-coil motifs (Stavolone et al. 2001; Hoh et al. 2010) which are bundles of two or more amphipathic helices, characterized by an heptad repeat of hydrophobic and hydrophilic amino acids.
Studies based on four Caulimovirus species suggest that VAPs assemble as a tetramer (Stavolone et al. 2001) and that the C-terminal region of the "Aphid transmission factor" (ATF) apparently interacts with VAP through the coiled-coil domains (Leh et al. 1999). VAP has been associated with the virion (Leh et al. 1999; Schmidt et al. 1994) but it is not essential for genome packaging (Kobayashi et al. 2002). Stavolone et al. (2005) suggest that VAP is not a scaffolding protein but a late addition during the assembly process that plays a role in the virus spread process. In fact, VAP is indispensable for spreading virus infection within the host plant (Stavolone et al. 2005) and may act as the arm of the virus particle anchored into the capsid shell (Leclerc et al. 2001). VAP also interacts with the C-terminal coiled coil domain of "Movement protein" (MOV), which oligomerizes as a parallel trimer that interacts with the VAP N-terminal coiled coil (Stavolone et al. 2005). This MOV-VAP interaction suggests that VAP is involved in virus movement (Stavolone et al. 2005). The figure below shows a three-dimensional structure of the virion-associated protein p3 from the Caulimovirus CaMV, according to the work of Hoh et al. 2010.